Permafrost: A Primer on Ice
2020-03-08
So, some of you may have wondered what that strange white fluffy stuff that occasionally falls from the sky is or what that weird cold stuff that your drink is filled with when you order a soda at a restaurant. Well, it’s something that’s known as “ice” and actually has some cool properties. I’m going to try and explain in this series, much of it adapted from Neil Davis’s Permafrost: A Guide to Frozen Ground in Transition.
Section 1: H2Whoa!
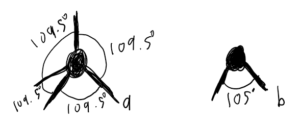
First off, H2O and what makes it special. If it sounds like I’m going through this quickly, it’s because I am, mostly just to get to Permafrost eventually. If you want more detail, I dunno, take a chem class. A lot of it comes down to the geometry of the molecule. The two Hydrogens are not just jutting off straight on either side, as they have to deal with the repulsion from the two sets of electron pairs coming off the Oxygen atom, ideally, they’d form a tetrahedron (Figure 1.1a) with 109.5° between each electron pair, but due to the size of the electron pairs, the Hydrogens are pushed to an angle of ~105° apart (Figure 1.1b).
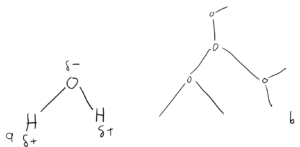
Now, the next bit of H2O’s specialness comes from the fact that Oxygen is a big ole electron hog, resulting in the molecule being “dipolar” with the Oxygen side of the atom being partially positive and the Hydrogen sides being partially negative (Figure 1.2a). This leads to something called ‘Hydrogen Bonding’ where the Hydrogens of one molecule are attracted to the Oxygens of another molecule (Figure 1.2b).
This Hydrogen bonding is really what makes things special. First, this leads to a high dielectric constant which leads to it being the ‘Universal Solvent’ because it can break up the ionic bonds such as those in common salt (NaCl). This leads to nutrients being dissolved in water, but, importantly, not bonded to water, meaning that they can be used by plants and the water stays the same chemically.
The Hydrogen bonding also increases the viscosity of the water (Which means it sticks together better). The viscosity of water is much higher than most other liquids and, importantly to ice and ice features, gets even more viscous as it cools (water at 0°C is approximately six times as viscous as water at 100°C)
Finally is Surface Tension and Adsorption. The Hydrogen bonding helps the water both stick together and stick to objects with Oxygen in their structures. This leads to its ability to ‘climb’ structures which will become very important when we get to ice structures.
Section 2: Ice Ice Baby (And Supercooling)
Now, quick quiz question. What temperature does water turn into Ice at (Assuming Earth’s surface pressure)? If you answered 0°C, you’re only part right. The correct answer is down to around -40°(F or C, they’re the same here) without Nucleation sites.
So, did your High School Chem teacher lie to you? How does the water bottle you left out freeze even though it only got down to -3°C? What does Nucleation Sites mean?
The answer lies in Supercooling, which is responsible for many of ice’s most interesting qualities. Now, if you’ve taken a Chem course, you should remember that just because something can happen or is favorable to happen, doesn’t mean it will happen. Your phone sitting on the edge of the table might look like it should fall, but could stay there forever until given a push. But your cracked phone lying on the ground doesn’t have this same ability to fall with just a push. This is the same as water at 0°C. The Water would be much more stable if it froze into ice, but, without a ‘push.’ This ‘push’ is the nucleation sites, which are any sort of thing that crystals can start growing on, a piece of dust, a grain of sand, or an already formed ice crystal. With pure, filtered water, you can get ice down to around -40° before the water will spontaneously form into ice. This can be summed up by Neil Davis’s Universal System Happiness Rule. Which is: Every System Is Happiest When It Contains The Least Possible Amount Of Free Energy. Some nucleation sites will cause ice to form at different temperatures, so it isn’t just a straight thing where a piece of dust causes ice to form, but I’ll go into that later.
And now one of the other cool features about ice: Why does ice float? For those who don’t know, this is odd. Most of the time a liquid is less dense than the solid form of the material. For example, Basalt magma has a density of around 2.65-2.80 g/cm^3, whereas Basalt itself will have a density of roughly 3+ g/cm^3. This means that basalt precipitating out of a magma will then sink into the magma. But with H2O, it is densest at around 4°C at 1 g/cm^3, with the density of ice being about 0.9 g/cm^3. For why this is, we are going to grossly oversimplify kinetic energy. Basically, the reason why the water molecules don’t just stick around is because they have some amount of kinetic energy, and this energy allows them to get closer than the hydrogen bond length (2.7e-8 cm), and as the water loses energy, the area of influence for each molecule shrinks, making the water molecule smaller, until Hydrogen Bonding can overcome. When the water finally freezes, the molecules all snap to the Hydrogen bond length of 2.7e-8 cm, This makes it less dense than the water and it will float. Ice also acts as a heat insulator, which is why lakes don’t usually freeze completely solid in the winter and why all life didn’t die out during a Snowball Earth.
Section 3: Minor Ice Features
So, to finish off this primer, we’re going to do a quick run through of a few small scale ice features, namely Pipkrakes, Hoar Frost, Rime Ice, Snowflakes (of course), and Icicles. I’ll just be giving them a quick paragraph and will likely come back to them later.


So to start I’m gonna talk about Pipkrakes (Figure 3.1,3.2), because they are the most adorable ice feature in the world. I just want to hug them so much. Pipkrakes area also known as Needle Ice, but, let’s be honest, Pipkrakes is a much more fun name, so I’ll continue with that. To put it simply, pipkrakes form when the temperature above ground is below freezing, the below ground temperatures are above freezing, and the soil has to be porous/permeable. The water in the soil is pulled upwards through capillary action and is frozen when it reaches the surface. As more water is drawn out the needles grow longer. This then loosens soil more than it was before, and smaller grains may lead to deflation of the soil (The removal of loose soil by wind). This cryoturbation can then start to sort the soil, as, while pipkrakes can lift larger pebbles, the needles mostly lift fine-grained material. I’ll talk about things like Frost flowers later, they are similar, but require me to think about biology, which is gross. #LifeLeavingTheOceansWasAMistake
Now, on to Hoar Frost and Rime Ice (Figure 3.3). And I hear you now, “Isn’t that the same thing as Hoar Frost?” And the answer is no, but they look the same if you aren’t looking too closely. The difference is, well, Hoar Frost is Frost and Rime Ice isn’t. Frost occurs when water vapor in above freezing air comes into contact with a below-freezing surface, and the water vapor deposits (goes directly from the gaseous state to the solid one) onto the surface. Hoar Frost forms during calm and clear nights. As the water vapor deposits on the surfaces it slowly grows it’s crystals, much in the same way dew is condensed, if there isn’t enough moisture, well, I’ll probably cover that in a post about farming down the line.

Now Rime Ice, as I said, is from supercooled water droplets and not vapor. Rime Ice occurs when a below freezing fog is blown across a landscape. You can have either “hard rime” or “soft rime” which occur under high wind conditions or lower wind conditions respectively. Hard Rime will tend to look more comb-like, whereas Soft Rime will appear spikier and is often confused with Hoar Frost. They’ll both appear on the windward side of the object.
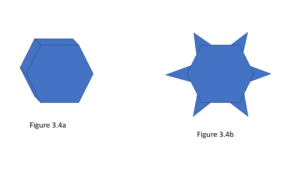
Then Snowflakes, what, let’s be honest, the ice thing you’re actually here for. They actually combine both supercooling and deposition. So first you have a cloud of supercooled water droplets waiting for a site to nucleate their crystal growth. While different specks of dust will nucleate the crystals at different temperatures, the average will be at -10°C. This frozen nucleus will likely be a hexagon due to the hexagonal crystal system of ice (Figure 3.4a). The water droplets surrounding the frozen nucleus will begin to evaporate due to the relatively high vapor pressure of water when compared to ice. This water vapor will then deposit onto the frozen nucleus and begin to grow the snowflake. Since this initial crystal is a hexagon, the corners stick out into more humid air and will start to collect deposited water vapor faster than the sides of the hexagon. This starts a feedback loop where the corners stick out more, they can access the more humid air, causing them to grow larger and to dehumidify the air that could have deposited ice onto the sides of the initial hexagon (Figure 3.4b). Now, there are other shapes of snowflakes, and I could go into much more detail, which I probably will someday, but this is already complicated for this primer piece.
To know more, check out SnowCrystals.com. I know, the name makes it sound like a Crystal Healer type, but the website is made by Doctor Kenneth Libbrecht, a CalTech Physics Professor who is widely known as an expert on Snowflakes. I got literally all my information on snowflakes from him.
And Icicles, these are quick because, well, these aren’t that hard. Basically, when ice melts, and starts to drip, in standard atmosphere, it will then quickly refreeze. As this happens over successive nights, the icicle grows longer. You can also have supercooled rain that drips and freezes or groundwater that’s dripping out the side of a rock face and then freezing.
Section 4: Where We’re Going from Here
First, if you do know about ice and water and whatnot and want to correct me, then do. One of the most important things to know about science is that no one knows everything, and it’s worth dropping someone a line when they’ve said something incorrect.
Second, this isn’t going to go in any particular order, mostly whatever strikes my fancy. If for some reason A: Someone is reading this, and B: you want me to talk about something specific, I guess I’ll do that, so drop me a line.
Third, let me know if you have any other geology topics you want me to talk about, I’m all ears. I’m just posting whatever I think is cool (Some pun intended).
Thanks for reading,
Daniel
Recent Comments